
With more manufacturers jumping on the hybrid bandwagon and Tesla hogging the electric-car limelight, car makers like Toyota and Hyundai, are putting some of their eggs in the hydrogen fuel cell basket to further boost their green credentials.
To the uninitiated, the thought of a family car being propelled by the stuff that saw the Hindenburg come crashing down to earth so spectacularly sounds like an alarming prospect.
But while hydrogen is flammable and explosive, it is safe to store in a car with a specially-designed tank, and thankfully doesn’t require a spark or compression to power the motor.
It’s also one of the most abundant elements in the universe.
Fuel cell vehicles are actually electric cars, but instead of using batteries to drive the motor, they create a chemical reaction between hydrogen and oxygen. This produces electricity that powers the motor, which drives the front wheels.
Hydrogen and oxygen are key ingredients of water (H20) which, along with heat, is the only by-product of fuel cell power generation; no carbon emissions and no apocalyptic explosions.
Producing power this way is also very efficient – around 60 percent more efficient – which, according to the US Department of Energy, is triple that of your average internal combustion engine.
So how does it work?
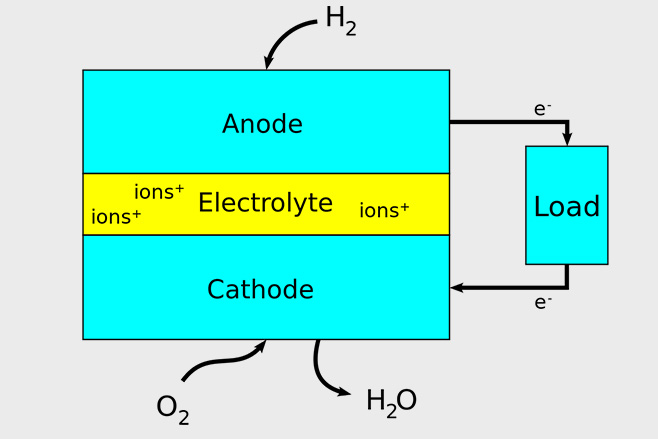
A fuel cell is like a battery that charges itself as long as there’s a steady supply of hydrogen from the fuel tank. The cell has positive and negative terminals which are separated by a thin plastic electrolyte like a sandwich.
Firstly, the hydrogen comes from the tank into the positive terminal (or anode) which is made of platinum. The oxygen is simply taken from the air and enters via the negative terminal (cathode). Platinum’s properties help speed up the chemical reaction, which sees the hydrogen atoms split in to hydrogen ions – which are atoms with electrons removed.
Next, the hydrogen ions are positively charged and attracted to the negative terminal, which is reached by passing through the electrolyte. Only the ions can pass through here.
Meanwhile, the electrons removed from the hydrogen atoms flow to and power the electric motor, which drives the wheels. After flowing through the motor they return to the cell via the negative terminal.
The ions and electrons then re-join with the oxygen, creating a chemical reaction that creates water which usually comes out of the exhaust pipe as water vapour (steam).
The question many people then ask is; if it’s so easy and hydrogen is an infinitely abundant power source that only emits water vapour, why aren’t we all driving hydrogen fuel cell cars?
It’s a bit of a chicken-egg thing. With so few hydrogen cars being made at present, the components remain expensive and hydrogen filling stations are very few and far between. As the demand for hydrogen-powered vehicles increases, it’s likely that there will be more filling stations introduced, but with so few filling stations, the demand isn’t quite there and hence the chicken and egg situation.
The benefits to the environment and the efficiency improvements highlight why manufacturers like Toyota and Hyundai are investing in bringing this technology to market, but while oil remains relatively cheap and conventionally-aspirated cars affordable, don’t hold your breath for the hydrogen-powered motoring revolution to arrive for a little while yet.
Want more? Check out our top 5 most fuel efficient cars.




